Breezula: Overview, Trial Results, and FDA Approval Date
Despite the fast advancement of most medical fields, the field of androgenetic alopecia has been in a dormant state for the entire 2000s. Since 1997, no new drug has been FDA-approved to treat the condition, which is estimated to affect 50 million men and 30 million women in the US alone.
Luckily for those affected, there’s a new drug on the horizon that’s patiently waiting for its FDA approval.
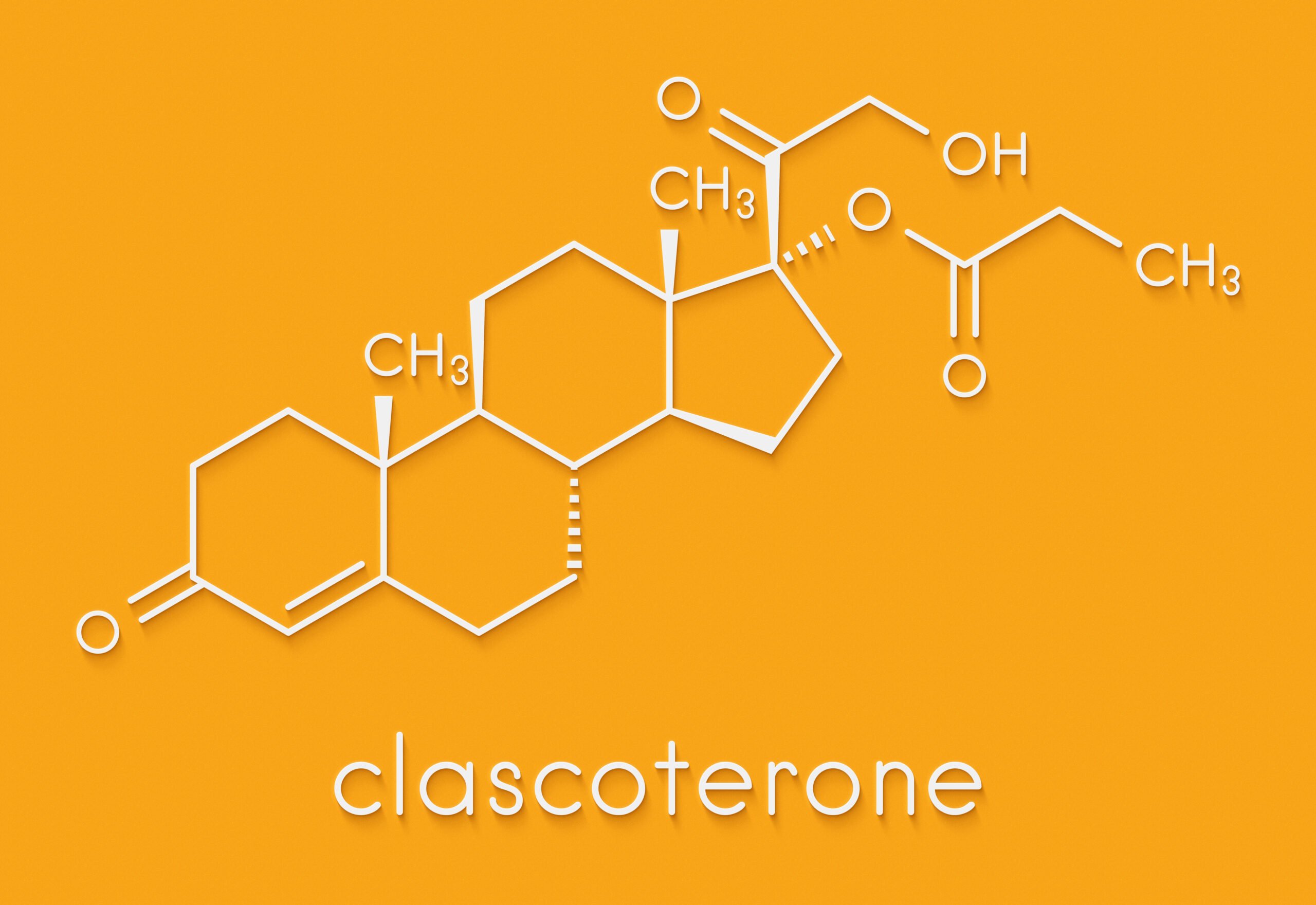
In 2019, an Italian pharmaceutical company called Cassiopea announced a new drug that presumably stops hair loss and regrows hair. They announced the drug under the name Clascoterone with the brand name Breezula.
Breezula reportedly inhibits the androgen receptors, stopping hair loss and encouraging the growth of new hair. It’s currently still under testing and isn’t available for purchase, so let’s see how much longer before it hits the market!
What Is Breezula?
Breezula is the brand name of Clascoterone, a new drug developed by Cassiopea, an Italian pharmaceutical company that specializes in medical dermatology.
Androgenetic alopecia often occurs because of your body’s excessive response to the production of androgens, the sex hormones that largely impact the hair cycle, and the rate at which your hair grows.
With an excessive reaction to these hormones, the hair follicles become overstimulated, causing hair to grow brittle and weak, often leading to pattern baldness and thinning hair.
Breezula works by blocking the androgen receptors in our nervous system, preventing hair loss. It’s a topical treatment, so it’s applied directly to the scalp like topical minoxidil.
Although some forms of the drug are approved for the treatment of acne vulgaris (Winlevi), it’s still not approved by the FDA as a hair loss treatment.
The Clacosterone drug is approved for acne treatment at a 1% concentration, but for hair loss, it’s tested at 2.5%, 5%, and 7.5% concentrations, so the testing process is vigorous and needs more time.
How Does Breezula Work?
The main idea behind Breezula is the same as finasteride—it blocks the androgens responsible for hair loss, but that’s putting it in the simplest way possible. In reality, the process is more complicated than that, but we’ll go into it bit by bit.
The Main Culprit: DHT
The main culprits behind hair loss and pattern baldness are androgens, a family of male hormones that are responsible for hair growth. The most influential hormone of this family is dihydrotestosterone or DHT as it’s more widely known.
DHT is the main reason behind countless hair-related issues, including androgenetic alopecia, hair loss, and hair thinning. It binds to the androgen receptors in your hair follicles and causes plenty of biochemical reactions that lead to the shrinking of these follicles.
Shrunk or unhealthy hair follicles lead to hair thinning and eventually hair loss in both men and women.
That’s why most hair growth products have the same target: blocking the DHT receptors to prevent the hormone from binding in the first place, thereby reversing hair loss.
The Breezula Effect
When you apply Breezula directly on your scalp, it rapidly metabolizes and binds to the androgen receptors in the hair follicles because it’s similar in form to DHT. However, it doesn’t activate the receptors, so the hair follicles remain unaffected.
At the same time, DHT can’t bind because the drug blocks the receptors, so the hair loss stops.
How Is Breezula Different From Finasteride?
Both Breezula and finasteride work in the same way by blocking the DHT effect. So, how is Breezule different from the already-established oral treatment that’s been FDA-approved for more than 25 years?
Finasteride does block the DHT effect, but it does so differently. Instead of binding to the androgen receptors, it targets the formation of DHT in the first place. It prevents testosterone from turning into DHT, leading to the reduction of the levels of DHT in your body.
Finasteride is scientifically more effective than Breezula because it’s already gone through all possible clinical trials and gained approval. It guarantees a positive outcome, which can’t be said about Breezula until it’s done being tested.
Despite that, Breezula may have leverage over finasteride because it doesn’t cause the same side effects. Finasteride is notorious for causing rapid mood swings and sexual dysfunction. As a topical treatment, Breezula doesn’t have any effect on the sex drive. It’s likely to come with side effects, but they’ll probably be skin-related like redness, itchiness, etc.
How Is the Breezula Testing Going?
Breezula has been undergoing testing for more than four years now. It’s currently being tested in both the US and Germany, and the results of the Phase III trial should be announced in 2024.
Until the official results are released, the drug can’t get approved yet, so let’s see how the testing process is going and when it’s expected to end:
The Phase II Trial
In 2019, Cassiopea published the results of a 12-month trial that was carried out on males suffering from androgenetic alopecia. The test included three concentrations of Breezula: 2.5%, 5%, and 7.5%.
The trial included 404 subjects between the ages of 18 and 55 with varying levels of alopecia. Some men applied the drug on the balding areas in their scalps for a whole year, while others applied the placebo.
The results showed positive outcomes in terms of hair loss prevention and growth of new hair. It also scored well in terms of local skin reactions and adverse effects.
The Phase III Trial
Based on the results of the Phase II trial, Cassipea originally chose the 7.5% concentration of Breezula for the Phase III studies, which were announced in June 2023. However, they later decided to change it, instead using 5% Breezula in larger volumes.
The studies were supposed to happen earlier but got delayed by the pandemic. They consist of a 6-month multicenter, prospective, randomized, double bind, vehicle-controlled study followed by a single-bind treatment for another 6 months.
This study will be significantly larger than the former, compassing a total of 1500 males, but until its results are announced, the drug can’t be approved for market use.
When Will Breezula Be FDA-Approved?
The FDA approval date is yet to be known, neither by Cassiopea nor by the FDA itself. For that to happen, the clinical trial of Breezula must end first with official results released.
After that, the review team of the FDA will analyze the data, thoroughly examine it, and reach a decision on whether its benefits outweigh its risks.
If the Phase III trial ends by June 2024 like the company intends it to, it may take around 6 months, give or take, to get the FDA approval. That means the drug will hopefully get its approval around the end of 2024 or the start of 2025.
Some drugs get approved in other countries, but not in the US, and some drugs get approved for other uses rather than hair loss treatment and then get used off-label. Breezula may be in a similar situation, depending on the outcome of the remaining trials.
What Are the Side Effects of Breezula?
Since Breezula’s trials aren’t done yet, its side effects aren’t revealed. After the Phase II trial ended, Cassiopea announced that there were no adverse effects directly related to the treatment drug. In other words, the drug is safe and not associated with risky side effects.
In a 2020 study of the Clascoterone drug, it was revealed that the side effects were mild and undangerous, but it was the acne-fighting drug rather than the hair loss version.
Until the drug is approved by the FDA for market use, we won’t know for sure the side effects because no official list will be released.
However, it’s likely that Breezula will have no severe side effects because of the way it works. When Clascoterone gets metabolized in our bodies, it produces cortexolone, a metabolite that occurs naturally in the human body and poses no risks. On top of that, Cassiopea revealed that Breezula doesn’t affect cortisol like some drugs do, so it doesn’t raise any flags.
One study was carried out on a similar cortexolone drug, and the potential side effects revealed were acne, hypersensitivity, and erythema. That still doesn’t mean Breezula will cause the same side effects, though.
Breezula Vs. Other Topical Treatments
Currently, the only drugs approved by the FDA for the treatment of hair loss are topical minoxidil and finasteride. However, dutasteride is also used as an off-label treatment, which means that it’s prescribed for a different condition but used to treat hair loss. Let’s see how these treatments work and how Breezula will compare:
Finasteride
Finasteride is often the primary treatment option for treating hair loss conditions, especially pattern baldness in men. It prevents further hair loss because it reduces the levels of DHT in your body by blocking the 5AR enzyme responsible for DHT synthesis.
Like minoxidil, finasteride is available in both topical and oral forms, and they both need a medical prescription. It’s also only prescribed for men because it causes plenty of side effects for women, including irregular menstruation, breast swelling, and increased body hair. Male users may suffer some side effects too, like erectile dysfunction and headaches, but they’re less severe.
Finasteride is likely the most similar treatment drug to Breezule out of this list. Both of them have a similar mechanism for stopping hair loss.
Minoxidil
Minoxidil is available in both oral and topical forms, and it’s shown excellent effectiveness in treating hair loss. You can buy topical minoxidil over the counter, while the oral form needs a medical prescription like finasteride. However, it has to be off-label because oral minoxidil isn’t FDA-approved for hair loss treatment.
Despite constantly being compared to finasteride, minoxidil works in a completely different way, which means it’s also different from the way Breezula works.
Minoxidil targets the blood vessels, dilating them to increase the blood flow to your hair follicles, thereby stimulating them and encouraging them to grow hair.
Its topical form has some mild side effects like dryness, scalp irritation, and flaking. Meanwhile, oral minoxidil may cause dizziness, eye puffiness, headaches, and insomnia, but it depends on every patient and his medical history.
Dutasteride
Dutasteride has the same treatment concept as finasteride. It works similarly by blocking the enzyme called 5-alpha reductase, which is responsible for producing DHT. However, instead of targeting the Type 2 isoenzyme like finasteride, it targets both Type 1 and Type 2 5AR.
Dutasteride has a more potent effect than finasteride, so it’s often used in severe cases where finasteride doesn’t score much progress. Besides, it targets the two isoenzymes, so it’s more efficient in reversing the effects of shrunk hair follicles.
In comparison, dutasteride can block more than 90% of the DHT synthesis process, while finasteride only blocks 70%. The drug can be both taken orally and applied topically.
Where Does Breezula Stand?
If we’re rating hair loss treatments from the most to the least effective, we’d say dutasteride is the most effective followed by finasteride with minoxidil in last place. Though not enough tests have been done on Breezula, we’d say it’ll have the same efficiency as finasteride in treating hair loss since it works the same way.
Minoxidil may be in last place when it comes to preventing hair loss, but it’s the most effective option when it comes to growing healthier hair. By stimulating the hair follicles, it encourages new hair to grow faster and thicker than before.
We don’t think Breezula will have the same effect in terms of hair regrowth, but we’re yet to see that.
Final Thoughts
Though Breezula is still under testing, there are high hopes already for potentially the first hair loss treatment to get approved by the FDA in two decades. The drug is currently only available in low concentrations to treat acne, but it’s yet to be available in its hair loss treatment forms.
Until the Phase III trial ends, we won’t know for sure whether Breezula will get the FDA approval, but we’re on the edge of our seats at the possibilities!